TIL - Melanoma Cancer Treatment
Feb 22, 2025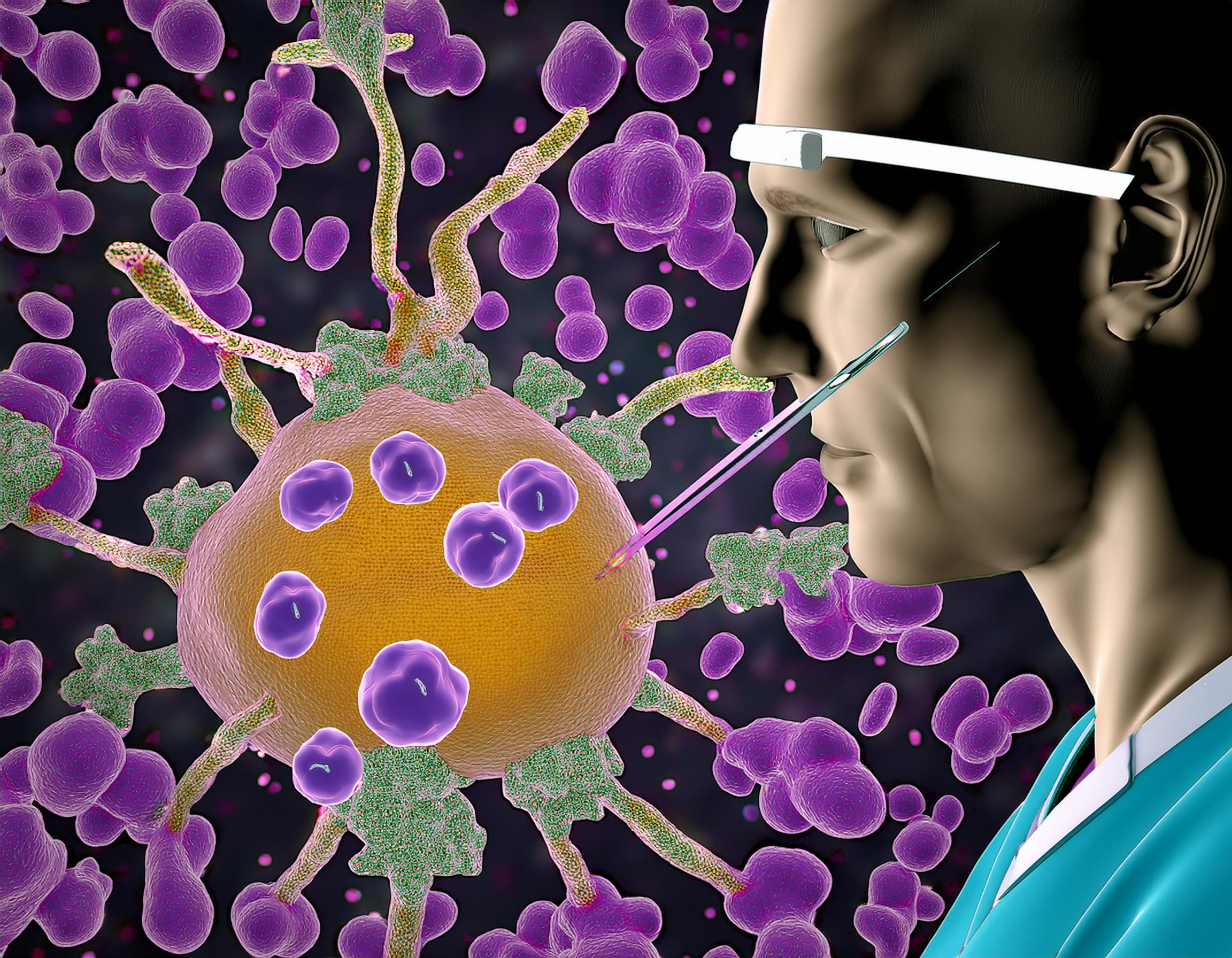
By Andrew Wilson
A good friend of mine's melanoma has returned. Two years ago he went through the newest immunotherapy treatment and we were all excited that this chapter was behind us. Unfortunately it was not.
With the bad news of the cancer's return, we are also heartened by a recently FDA approved therapy, TIL (Tumor-Infiltrating Lymphocyte) and the program being let by Dr. Sylvia Lee at the Fred Hutchinson Cancer Center in Seattle.
Here is some more information on TIL therapy.
Tumor-Infiltrating Lymphocyte (TIL) therapy is an advanced form of immunotherapy designed to harness a patient’s own immune system to combat melanoma. This approach involves extracting TILs—immune cells that have naturally migrated into the tumor—from a patient’s tumor sample. These cells are then expanded and activated in a laboratory setting before being reintroduced into the patient’s bloodstream, where they target and attack cancer cells. To enhance the effectiveness of the infused TILs, patients often receive interleukin-2 (IL-2) therapy, a cytokine that promotes T-cell growth and activity. IL-2 can be administered systemically or directly into the tumor, depending on the treatment protocol.
Dr. Sylvia Lee, an oncologist at Fred Hutchinson Cancer Center, specializes in TIL therapy for melanoma and lung cancers. She leads the TIL Therapy Program at Fred Hutch, one of only four centers in the United States offering clinical trials in this area. Dr. Lee’s work focuses on extracting TILs from patients’ tumors, expanding them in the lab, and reinfusing them to bolster the body’s natural defenses against cancer. Her research aims to refine this process to improve patient outcomes and expand the applicability of TIL therapy to other cancer types. 
Clinical studies have demonstrated that TIL therapy can be effective, particularly in patients with metastatic melanoma who have not responded to other treatments. Response rates in these heavily pretreated patients range from 30% to 50%, with some experiencing complete and long-lasting remissions. For instance, a study reported that among patients treated with TILs, 20% achieved a durable complete response. 
However, TIL therapy is associated with significant side effects, primarily due to the IL-2 administration and the preparative regimens used to enhance TIL efficacy. Common side effects include flu-like symptoms, nausea, low blood pressure, and more severe complications such as vascular leak syndrome, which can lead to life-threatening conditions like pulmonary or brain edema. These adverse effects typically occur within the first two to three weeks post-treatment, with few long-term side effects observed. 
In summary, TIL therapy combined with IL-2 offers a promising treatment avenue for patients with advanced melanoma, especially those who have exhausted other options. Under the guidance of experts like Dr. Sylvia Lee, ongoing research continues to optimize this therapy, aiming to enhance its efficacy while managing its associated risks.
Get the Free Trial to Try Memios
Categories
All Categories 1 corinthians 13:4-7 16/8 fasting 4-7-8 method a-z food additives acesulfame k acesulfame potassium achieve goals achievement active listening active listening skills active listening techniques ai wellness alkalizing alternate-day fasting american food chemicals analyze patterns anti-aging anti-inflammatory anti-inflammatory diet anti-inflammatory foods antibacterial antioxidant anxiet spikes anxiety anxiety ina digital world anxiety management arthritis artificial colors artificial sweeteners aspartame athletic performance autophagy awareness balanced diet balanced life better boyfriend better sleep bha bht bible explorer bible study biblical principles biblical teachings bile blood circulation blue 1 blue 2 boosted immune system boundaries brain fog brain health brain-enhancing breaking bad habits breastcancer broken relationships brown fat buddhist prayer budgeting budgeting strategies building a strong faith building good habits business planning business strategy butylated hydroxyanisole butylated hydroxytoluene caffeine calm mind calorie burning cancer cancer therapy cancer treatment cancerexercise canola oil cardio cardiomyopathy screening adjustments cardiovascular disease care models career choices career coaching career development career growth career success career support carrageenan cayenne cayenne powder chaga chemotherapy childhood cancer survivorship children’s oncology group (cog) children’s oncology group (cog) long-term follow-up guidelines cholesterol christian christian habits christian leadership christian meditation christian prayer christian purpose christian values christianliving chronic diseases chronic inflammation citric acid coaching cognitive function cognitive reframing cold exposure cold flu cold plunge cold plunging colossians colossians 3:13 communicaiton skills communication community community engagement community service compassionate partner comprehensive long-term care concentration confidence conflict resolution conflict resolution strategies congestion coping mechanisms cordyceps cordyceps militaris corinthians corn syrup cough counseling crisis management curcumin daily meditation daily mindfulness daily planning daily prayer daily prayer journal dailyhabits datem decision making decision-making deep breathing depression detoxification devotional tracking diacetyl tartaric acid esters of mono- and diglycerides digestion digestive aid digital connections digital curfew digital detox digital health tools digital journaling disagreement disodium guanylate disodium inosinate disrespect disussion dopamine dr. sylvia lee duckabush mushrooms echocardiograms education education support ei eisenhower matrix emotional bonds emotional intelligence emotional intelligence development emotional quotient emotional reactivity emotional regulation emotional regulation strategies emotional stability emotional support emotional triggers emotional wellness emotionalintelligence emotions empathetic leadership empathy empowering survivors endorphins endurance energy energy booster energy-boosting energyboost energymanagement enhance rest enhanced mood entrepreneurship eq ethical decision-making ethical living ethical-driven living exercise routines extracts faith and health faith journey faith networks faith-based communities faith-based decision-making faith-based habits faith-based journaling faith-based learning faith-driven mentors faithandwellness faithbasedliving faithscience family guidance fasting fasting and fitness fasting and mental clarity fasting benefits fasting for longevity fat burn fear fermented foods financial growth financial planning financial stewardship find peace fitness fitness coaching fitness plans fitness routine flu flu prevention focus food additive health effects food additives food colorings food emulsifiers food preservative list food stabilizers food thickeners foregiveness forgiveness fred hutchinson cancer center ganoderma lucidum gellan gum general wellness genetic cancer genetic cancer predisposition screening ginerol ginger ginger lemon shot ginger root ginger shot global survivorship trends glucose glycerin in food goal achievement goal setting goals grace gratefulheart gratitude gratitude exercises gratitude journal gratitude journaling green 3 growth journey growth mindset guar gum guided meditation gut health gut microbiome gut-brain connection habit formation habit forming habit tracking habitformation happiness harmful food additives health and fitness habits health monitoring healthandwellness healthy diet healthy eating healthy fasting habits healthy habits healthy lifestyle healthy skin healthyeating healthyliving heated discussion hericium erinaceus hidden ingredients in food high-fructose corn syrup hindu prayer holistic life optimization holistic support holistic wellness honey hostility how to fast humility hydrogenated oils il-2 immune boosting immune cells immune health immune support immune-based therapy immunity immunotherapy improved circulation improved mood improvement unlimited in-person interactions increased metabolism indigestion inflammation inflammation and mental health inflammation and productivity inflammation and spirituality inflammation causes inflammation cures inflammation supplements influence inner peace inner peach inonotus obliquus insulin sensitivity intelligence-supported major life decisions intelligent bible study intelligent career coaching intelligent progress tracking intelligent reminders intelligent speech analysis intention setting interfaith prayer examples interleukin-2 intermittent fasting interpersonal stress james 1:19 jay harness md jay k harness jay k harness md jewish prayer joint health joint pain joint stiffness journaling journaling apps journaling systems late-onset complications leadership learning opportunities lentinula edodes libido life balance life challenges life decisions life planning life purpose lifelong learning lion's mane liver detoxify liver function liver health long-term aspirations longevity love lung function magnesium major life decisions manage anxiety manage workload marriage marriage guidance md meal planning mediation medicinal mushroom meditation melanoma memios memios wellness memory mental clarity mental health mental health support mental imagery mental resilience mental well-being mental wellness mentalclarity mentalhealth mentalwellness mentorship metabolic health metastatic melanoma mindful eating mindful habits mindful living mindfulliving mindfulness mindfulness practices misunderstanding modified food starch money-related stress mono- and diglycerides monosodium glutamate morning routine motivation motivational content motivational mentors msg multitasking muscle recovery muscle soreness mushroom coffee mushroom extract mushroom tea mushroom tincture mushrooms muslim prayer natural remedies for inflammation nature reviews clinical oncology nature-based activities navigate stress nerve damage neurocognitive impairments neuroscience of gratitude neuroscience of habits nitrates nitrites norepinephrine nurture relationships nutrition nutrition for brain function nutritional guidance omad omega-3 open-mindedness optimal health overcoming failure painmanagement paper journaling partially hydrogenated oils partner pediatric cancer treatment performance anxiety performanceenhancement personal development personal goals personal growth personal progress personal resilience personal values personaldevelopment personalized care personalized fitness plans personalized learning paths personalized medicine ph levels philipians philippians 2:3-4 physical health polysorbate 60 polysorbate 65 polysorbate 80 positive mindset positive psychology positive routines positive work enviornment positivity potassium bromate potassium nitrate potassium nitrite prayer prayer and meditation prayer and mental health prayer benefits prayer for emotional healing prayer journal prayer tracking prebiotics precision medicine premature mortality probiotics problem-solving problem-solving skills processed food ingredients productivity professional goals propyl gallate propylene glycol proverbs proverbs 3:5-6 psychological distress psychology of habits psychosocial support ptsd public speaking purpose driven living purpose-driven living purposeful living purposefulliving quality of life quantity of life radiation raw honey reality fuel recalibrate attention spans red 40 reduce bloating reduce inflammation reduce nausea reduce stress reduce uncertainty reduced anxiety reduced inflamation reduced stress reduces cholesterol reframe reishi relational anxiety relationship building relationship-building relationships relaxation relaxation techniques religious wellness resilence resilience respect reward reward yourself risk-based screening programs schedule management science of prayer screen fatigue scripture secondary cancers seed oil additives seed oil health risks self-affirmations self-awareness self-growth self-improvement self-reflection self-regulation selfimprovement serving others shared decision-making shared wisdom shiitake side affects single-tasking skin health sleep sleep optimization sleep quality sleep tracking sleepandproductivity sleepoptimization smoothie social engagement social skills social well-being sodium benzoate sodium nitrate sodium nitrite sodium phosphates sorbitol sore throat soy lecithin soybean oil specialized survivorship care spiritual discipline spiritual growth spiritual health spiritual mindfulness spiritualgrowth spiritualhealth splenda stamina stress stress management stress management strategies stress reduction stress relief stressrelief success mindset sucralose sugar sunflower oil superfoods supplements support networks supportive partner surrender surveillance for novel therapies surveillance strategies survivorship care survivorship clinics survivorship research targeted therapy tbhq telemedicine tert-butylhydroquinone til time blocking time management time-blocking tinctures titanium dioxide tocopherols tonic toxins trametes versicolor trans fats trust tumor-infiltrating lymphocyte turkey tail turmeric turmertic powder understanding updated vaccination guidelines vaccination guidelines values alignment values-driven decision making vascular leak syndrome vegetable oil vision boards vision mapping visualization visualization and success visualization for career growth visualization for health visualization for success visualization practice visualization techniques vitamin c wealth wealth management weekly wellness plan weight loss fasting well-being wellness wellness communities wellness habits wellnessjourney wellnesstools work-life balance workload workout regimen workplace influence workplace leadership workplace relationships worship xanthan gum yellow 5 yellow 6 yoga
